

There were multiples number ratios in various compounds like oxygen compound.
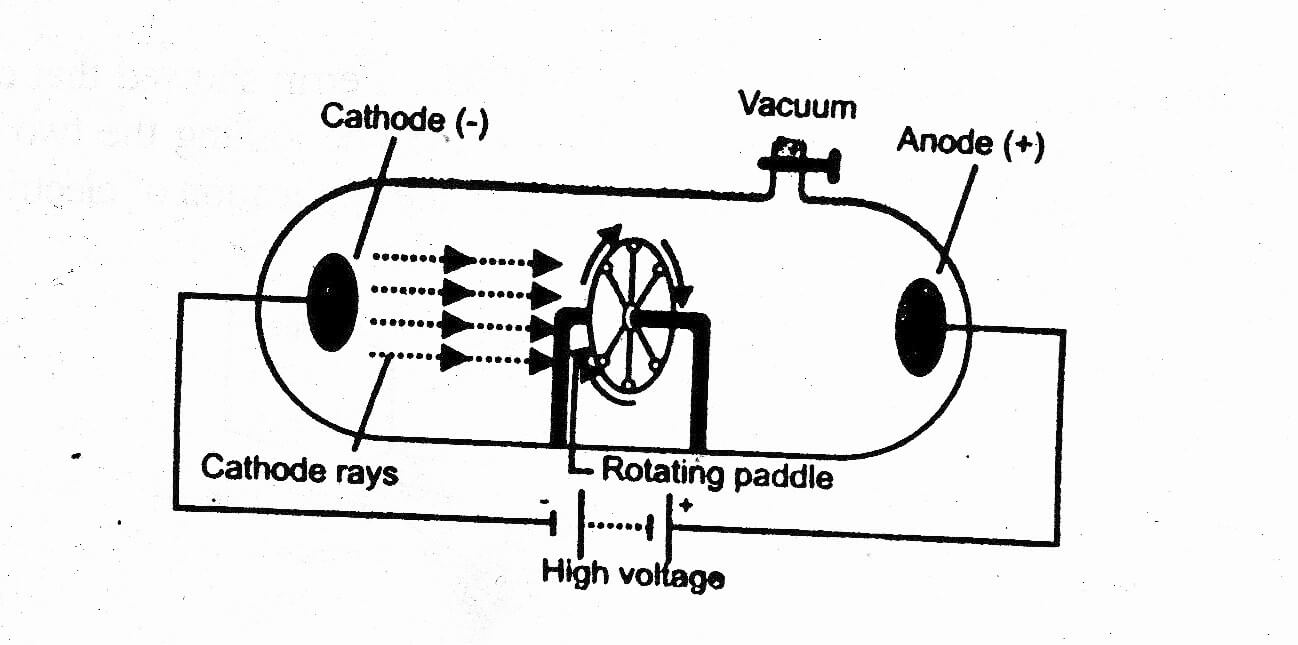
While atoms in the different element will have the different characteristic from one to another. Every single atoms in oxygen is same to another. Dalton believed that all atoms in the same element must have same weights. The weight of atom determines the characteristic of atom.He used the Antoine Lavoisier theory to support this point The atoms in element can not be created, destroyed, divided or transformed. Atom can not be destructed and changed.He also said that atoms have various motions He belived that atoms has the small shape and solid spheres form. All mater contains very small particles which are called atoms.His theory has five basic principle as following points: John Dalton conducted the atomic theory called the five postulates of John Dalton. In 1803, John Dalton released his atomic theory which will then become one of the most useful theory in chemistry. Prior Study by Dalton that Triggered JJ Thomson Scientists Who Contributed to The Atomic Theory.The technology for these displays is much different than that for CRTs. Notebook computers typically use liquid crystal display. The most common specification for CRT displays is known as SVGA (Super Video Graphics Array). In computer systems, there are several display modes, or sets of specifications according to which the CRT operates. The CRT thus produces three overlapping images: one in red (R), one in green (G), and one in blue (B). These devices have three electron guns, one for the primary color red, one for the primary color green, and one for the primary color blue. However, virtually all CRTs today render color images. This is typical of a monochrome, or single-color, CRT. The illustration shows only one electron gun. But the scanning takes place at such a rapid rate that your eye sees a constant image over the entire screen. As viewed from the front of the CRT, the spot moves in a pattern similar to the way your eyes move when you read a single-column page of text.
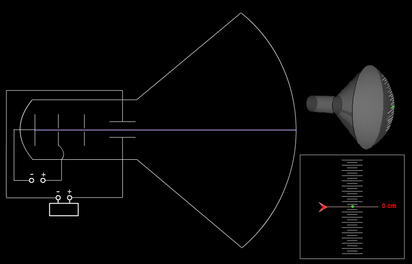
This causes the spot to race across the screen from right to left, and from top to bottom, in a sequence of horizontal lines called the raster. To produce an image on the screen, complex signals are applied to the deflecting coils, and also to the apparatus that controls the intensity of the electron beam. The electron beam produces a tiny, bright visible spot when it strikes the phosphor-coated screen. There are two sets of deflecting coils: horizontal and vertical.(In the illustration, only one set of coils is shown for simplicity.) The intensity of the beam can be varied. Deflecting coils produce an extremely low frequency electromagnetic field that allows for constant adjustment of the direction of the electron beam.
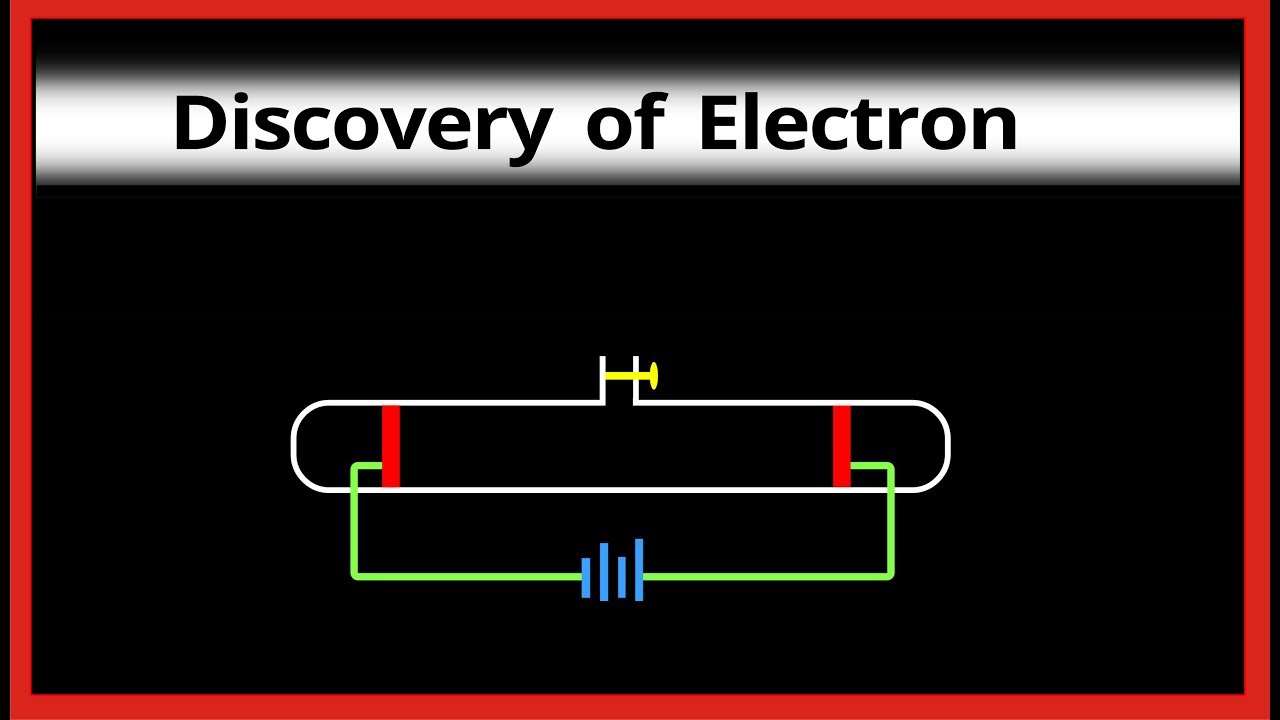
The electron gun generates an arrow beam of electrons. The CRT in a computer display is similar to the "picture tube" in a television receiver.Ī cathode-ray tube consists of several basic components, as illustrated below. Most desktop computer displays make use of CRTs. A cathode-ray tube (CRT) is a specialized vacuum tube in which images are produced when an electron beam strikes a phosphorescent surface.


 0 kommentar(er)
0 kommentar(er)
